Abbott BinaxNOW COVID-19 Ag CARD HOME TEST KIT Ohio User Guide. Instructions and is intended to provide helpful testing tips when using the Abbott BinaxNOW COVID-19 Ag Card test.

Binaxnow Covid Test At Home With Report For Travel Meenta
NAVICA displays results from the 15-minute Abbott BinaxNOW COVID-19 Ag Card a rapid antigen test to help you and others make informed decisions.
Abbott covid 19 ag test instructions. WWWGLOBALPOINTOFCAREABBOTT Product not available in all countries. April 6 2021. COVID-19 Ag Test TM 2C 36F 30C 86F Test device individually in a foil pouch with desiccant Extraction buff er tube Nozzle cap Sterile swab Instructions for use Carefully read instructions for using the STANDARD Q COVID-19 Ag Test.
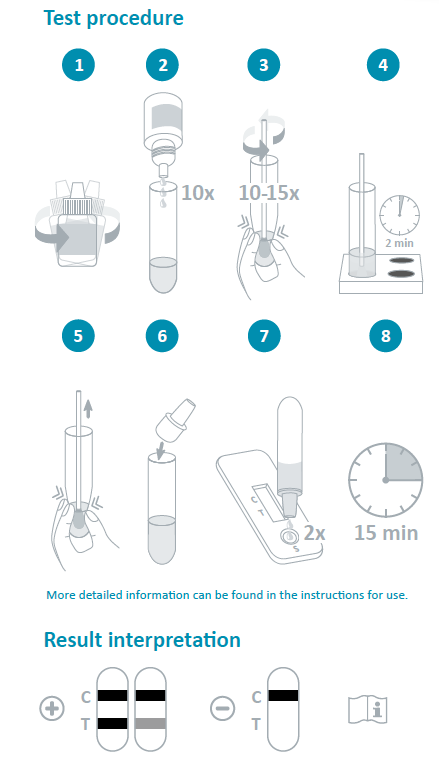
The product may be used in any laboratory and non-laboratory environment that meets the requirements specified in the Instructions for Use and local regulation. COVID-19 Ag Rapid Test Device is for professional use only and is intended to be used as an aid in the diagnosis of SARS-CoV-2 infection. To ensure accurate performance of this test please refer to the package insert or Instructions for Use for complete details on how to perform the test.
This guidance describes the conditions for. The Abbott BinaxNOW COVID-19 Ag Card is a rapid antigen test in a card format that detects SARS-CoV-2 protein antigens present in a nasal swab specimen. 4 BinaxNOW COVID-19 Ag Card Home Test Instructions for Use 7 Swab Right Nostril A.
The NAVICA app is your pass to navigating daily life in a new normal. WWWGLOBALPOINTOFCAREABBOTT In medical diagnosis sensitivity is the ability of a test to correctly identify those with the disease. Utilize product as outlined in the BinaxNOW COVID-19 Ag Card Instructions for Use.
Test Device Instructions for Use Tube Blue Cap. The BinaxNOW COVID-19 Ag Card can be used to test anterior nasal nares swab samples. It has been authorized by the FDA under an emergency use.
The tests can be used in point-of-care settings and at home with an online service provided by eMed. EASY-TO-USE SIMPLE PROCEDURE CONTACT YOUR LOCAL REPRESENTATIVE TODAY. It has been authorized by the FDA under an emergency use authorization for use by authorized laboratories and patient care settings.
Results from the simple nasal swab are available in 15 minutes through testing individuals suspected of COVID-19. The test has been authorized only for the detection of proteins from SARS-CoV-2 not for any other viruses or pathogens and is only. This test is used on our ID NOW instrument.
For use with nasal swab specimens. Abbott BinaxNOW Ag Test Card Guidance UPDATED. The test provides preliminary test results.
For more information on ID NOW check out this article. February 12 2021 Version Introduction. The BinaxNOW COVID-19 Ag Test Card EUA has not been FDA cleared or approved.
Check the test device and the desiccant pack in the foil pouch. Testing is done at the point of. Our rapid antigen test BinaxNOW COVID-19 Ag Card provides results in 15 minutes when used to test individuals suspected of COVID-19.
Organizations will be able to view and verify a persons digital NAVICA Pass on their mobile device to facilitate smoother entry into facilities along with. The State of Maine is distributing rapid point-of-care POC antigen Ag tests Abbott BinaxNOW COVID-19 Ag Card Ag Cards. This is a diagnostic test designed for use in individuals suspected of having COVID-19 by their healthcare provider within the first 7 days of symptom onset.
The Panbio COVID-19 Antigen Self-Test has been shown in clinical evaluations performed by professional health care persons to correctly identify 998 403 out of 404 of SARS- CoV-2 negative nasal samples with a confidence interval of 986 to 1000 known as test. Failure to follow the instructions for test procedure and interpretation of test results may adversely affect test. The BinaxNOW COVID-19 Ag Test Card EUA has not been FDA cleared or approved.
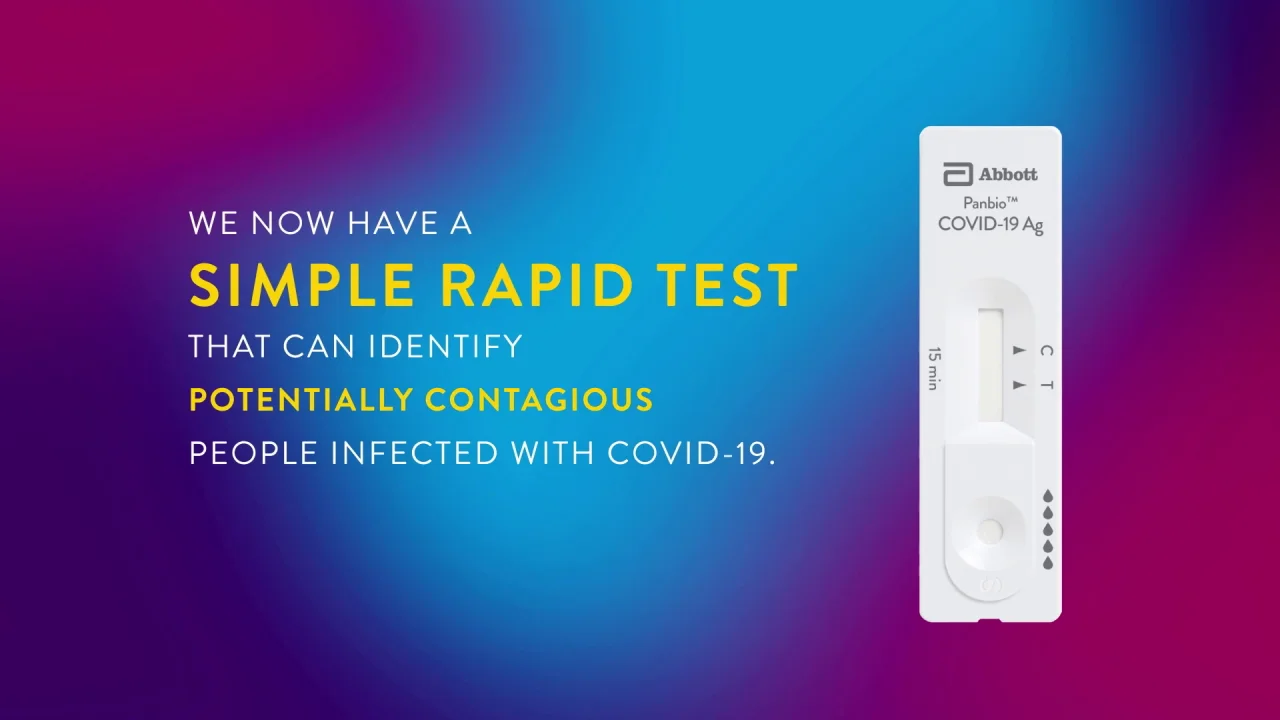
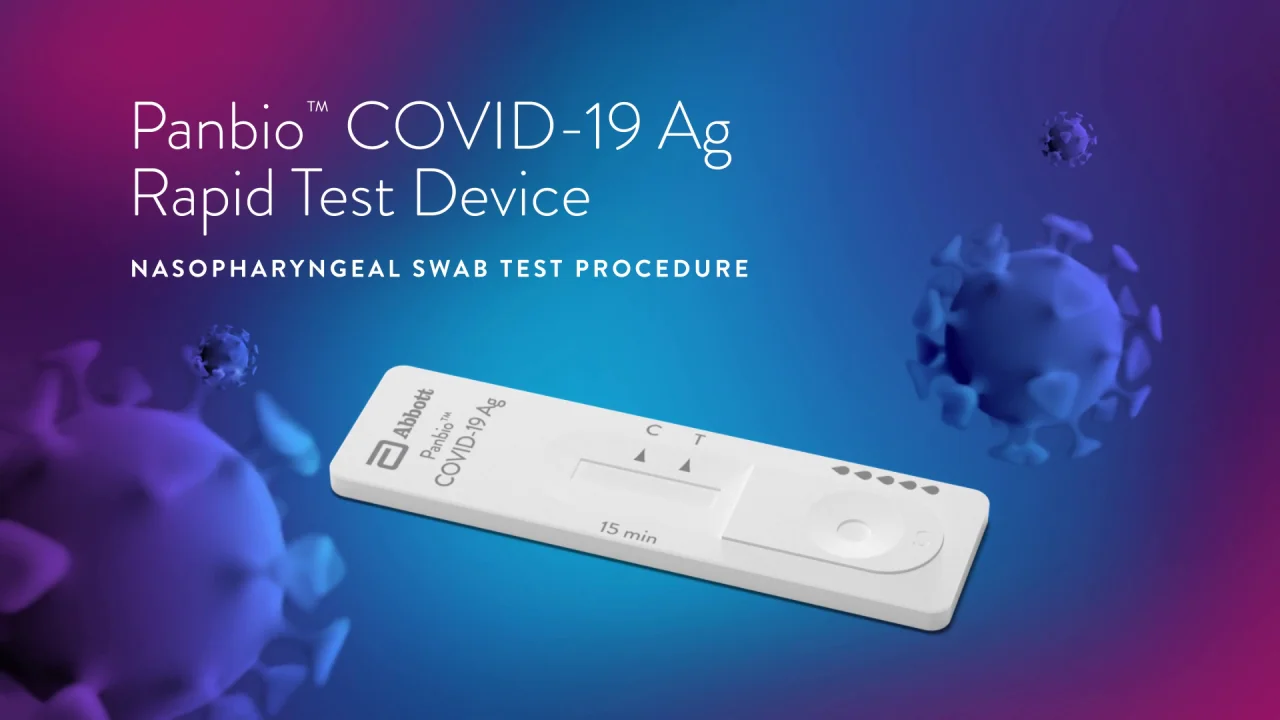
Remove swab and insert it into right nostril. Firmly brush against insides of nostril in a circular motion 5 times. PANBIO COVID-19 Ag RAPID TEST DEVICE NASAL SWAB.
Panbio COVID-19 Ag Rapid Test Device is an in vitro diagnostic rapid test for the qualitative detection of SARS-CoV-2 antigen Ag in human nasal swab. Abbotts BinaxNOW COVID-19 Ag Card test can identify these antigens which are typically detected after symptoms start. Self-test via video call using the BinaxNOW COVID-19 Ag Card Home Test and Abbotts complementary NAVICA mobile app to enable the testing process and display BinaxNOW COVID-19 test results.
Page 1 of 14 BINAXNOW COVID-19 AG CARD PN 195 -000 INSTRUCTIONS FOR USE BinaxNOWTM COVID-19 Ag CARD. For Use Under an Emergency Use Authorization EUA Only. 10 TEST PACK 20 TEST PACK 1 TEST PACK CATALOG NUMBER.
Kentucky Department for Public Health and Abbott Laboratories December 14 2020 100 - 215 PM. The BinaxNOW COVID-19 Ag Card 2 Home Test is a lateral flow immunoassay intended for the qualitative detection of nucleocapsid protein antigen from SARS-CoV-2 in direct anterior nasal nares swabs from individuals with or without symptoms or other epidemiological reasons to suspect COVID-19 infection when tested twice over three days with at. The BinaxNOW COVID-19 Ag Card is a rapid antigen test and both the Food Drug.
Not approved for sale in the USA. Abbott Diagnostics Scarborough Inc. BinaxNOW COVID-19 Ag CARD HOME TEST KIT Getting Started ADownload the NAVICA app from the Apple App Store or Google Play Store on your smartphone or tablet BLogin or create a NAVICA account create a.
THE PROFESSIONAL USE PANBIO COVID-19 AG RAPID TEST DEVICE. CONTACT YOUR LOCAL REPRESENTATIVE TODAY. Our rapid molecular point-of-care test detects COVID-19 in 13 minutes or less.

Abbott Panbio Covid 19 Antigen Rapid Test Nasal Meinarztbedarf Com

Abbott Launches Rapid Portable Antigen Test Increasing Covid 19 Testing Capacity In France 2020 09 18 Bioworld
.jpg)
Panbio Nasaler Covid 19 Ag Schnelltest

How To A Guide For The Binaxnow Covid 19 Self Test Youtube
Panbio Covid 19 Ag Rapid Point Of Care Abbott
Https Extranet Who Int Pqweb Sites Default Files Documents Eul 0587 032 00 Panbio Agrapidtestdevice Nasal V3 Pdf

Panbio Covid 19 Ag Rapid Point Of Care Abbott
Https Extranet Who Int Pqweb Sites Default Files Documents Eul 0587 032 00 Panbio Agrapidtestdevice Nasal V3 Pdf

Abbott Panbio Covid 19 Antigen Rapid Test Nasal Meinarztbedarf Com

Corona Rapid Antigen Test Cegat Gmbh

Panbio Covid 19 Ag Rapid Point Of Care Abbott

Nein Abstrichstabchen Von Corona Schnelltests Sind Nicht Verseucht
Https Extranet Who Int Pqweb Sites Default Files Documents Eul 0587 032 00 Panbio Agrapidtestdevice Nasal V3 Pdf
Nadal Covid 19 Antigen Schnelltest 20 Corona Tests Nasal Medizinischer Fachhandel

Panbio Covid 19 Ag Rapid Test Device Abbott U K

Abbott S Binaxnow Covid 19 Ag At Home Test Kit 6 Pack
Panbio Covid 19 Ag Rapid Point Of Care Abbott
Abbott Binaxnow Rapid Antigen Test And Navica Mobile App Facebook
Https Extranet Who Int Pqweb Sites Default Files Documents Eul 0587 032 00 Panbio Agrapidtestdevice Nasal V3 Pdf
Abbott Covid 19 Ag Test Instructions. There are any Abbott Covid 19 Ag Test Instructions in here.
Search This Blog
Blog Archive
- July 2023 (1)
- November 2022 (12)
- August 2022 (7)
- February 2022 (3)
- August 2021 (55)
Labels
- 2020
- 2021
- 25th
- abbott
- amendment
- american
- arsenal
- article
- B. IndonesiaSekolah Dasar
- B. inggrisSekolah Dasar
- beach
- binaxnowtm
- brisbane
- cabinet
- card
- chelsea
- child
- coast
- coloring
- constitution
- control
- covid
- definition
- device
- first
- GeografiSekolah Menengah Pertama
- highlights
- IPSSekolah Dasar
- kids
- lck
- lck 2021
- lck cl
- lck english
- lck global
- lck highlights 2021
- lck live
- lck summer
- lck summer 2021
- lck vs lpl
- lions
- MatematikaSekolah Dasar
- ngày thành lập công an nhân dân việt nam
- ngày truyền thống công an nhân dân
- nghệ sĩ saxophone trần mạnh tuấn
- odds
- page
- playing
- predictions
- premier
- rapid
- sand
- SeniSekolah Menengah Atas
- significance
- test
- tickets
- votes
- wallpaper
- west
- τοτεναμ παοκ 1-2
- τοτεναμ παοκ 1-2 κερκιδα
- азиза
- замуж
- зенит
- инстаграм
- матчи
- молодости
- муж
- ноги
- певица
- песни
- петербург
- санкт
- спартак
- тальков
- умарова
- фото
